FDA won't comment on status of Emergency Use Authorizations for two antibody treatments
Por um escritor misterioso
Last updated 21 setembro 2024

The US Food and Drug Administration told CNN Thursday morning that the agency doesn’t have any comments on the applications for Emergency Use Authorizations for Eli Lilly and Regeneron antibody treatments.
The coronavirus pandemic has brought countries to a standstill. In many places, as countries reopen, Covid-19 cases are on the rise. Follow here for the latest.
The coronavirus pandemic has brought countries to a standstill. In many places, as countries reopen, Covid-19 cases are on the rise. Follow here for the latest.

Biomedicines, Free Full-Text
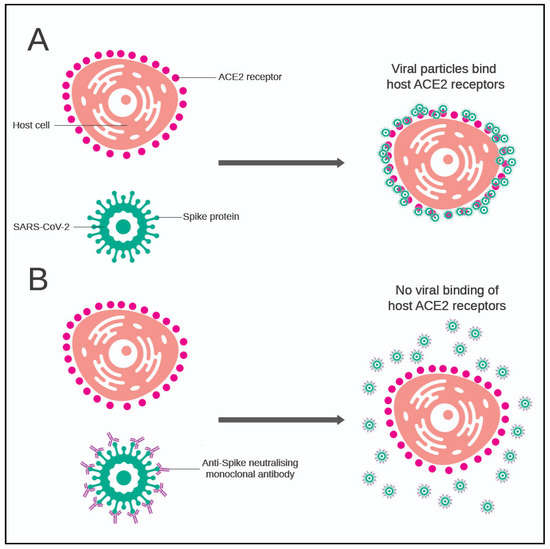
COVID, Free Full-Text

Federal Register :: Authorizations of Emergency Use of Certain

IJMS, Free Full-Text

Federal Register :: Authorizations of Emergency Use of Certain

Eli Lilly's Antibody Treatment Gets Emergency F.D.A. Approval
FDA Open Meeting on Johnson & Johnson COVID-19 Vaccine, Part 4

F.D.A. Allows Expanded Use of Convalescent Plasma to Treat

Federal Register :: Authorizations of Emergency Use of Certain

Gov. John Bel Edwards - These safe and effective COVID vaccines

Merck's New Covid Drug Molnupiravir Gets FDA Approval In Time for

Federal Register :: Authorizations of Emergency Use of Certain

FDA announces emergency authorization of plasma treatment for COVID-19
Recomendado para você
você pode gostar