Hep B biotech Antios closed after FDA hold proved insurmountable
Por um escritor misterioso
Last updated 12 novembro 2024

Viral disease biotech Antios Therapeutics shut down earlier this year after an FDA hold on its lead hepatitis B therapy due to a serious adverse event proved insurmountable. | Viral disease biotech Antios Therapeutics shut down earlier this year after an FDA hold on its lead hepatitis B therapy due to a serious adverse event proved insurmountable.

Volume 21, Issue 5 by Western Journal of Emergency Medicine - Issuu

Bone Scaffolds: An Incorporation of Biomaterials, Cells, and
Hep B biotech Antios closed after FDA hold proved insurmountable

Arbutus' oral checkpoint inhibitor slapped with clinical hold

Landon Loving sur LinkedIn : GSK's $100M ADC bet in doubt after
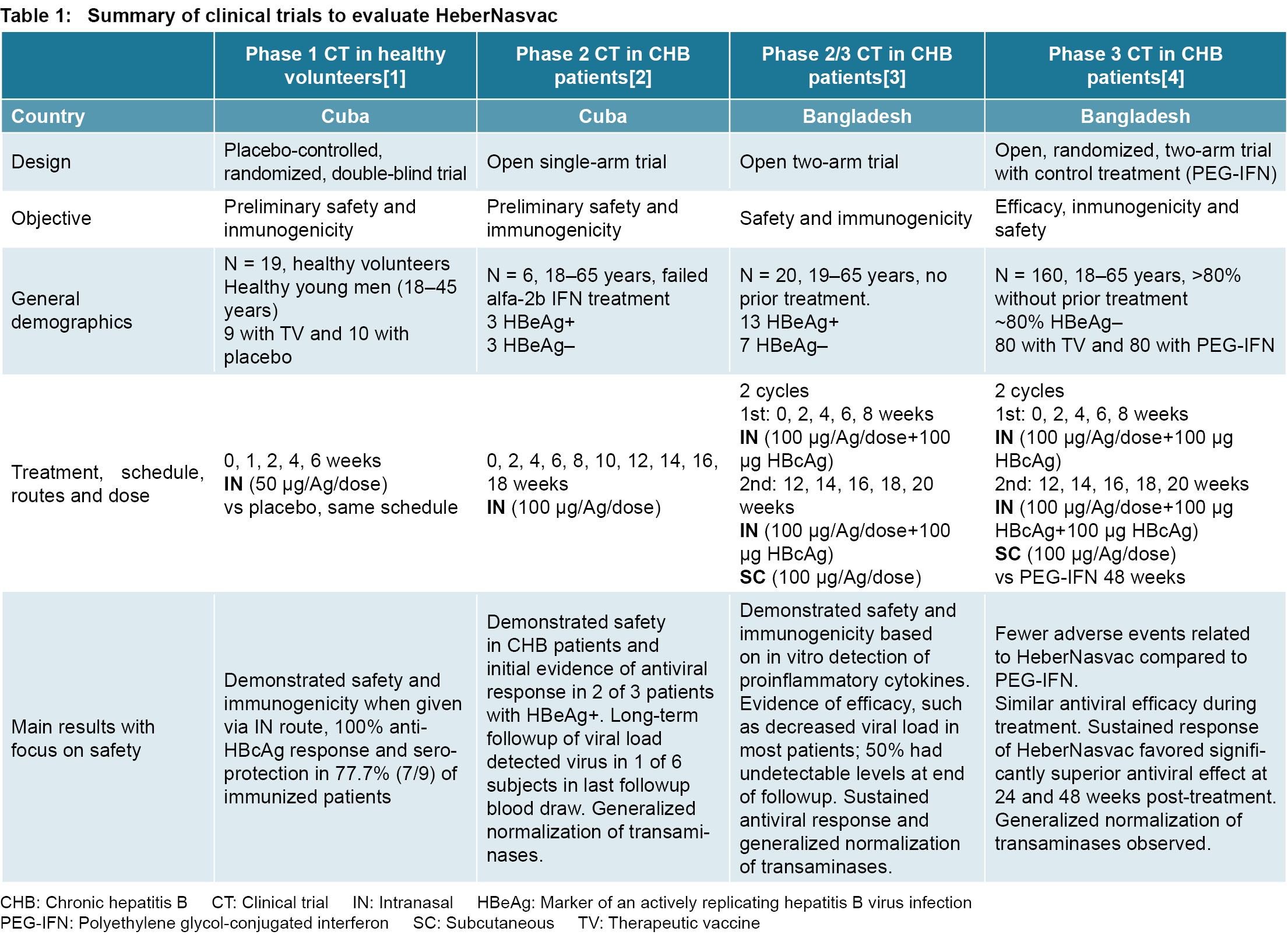
MEDICC Review Cuban Prophylactic and Therapeutic Vaccines for

Microfluidic Formulation of Topological Hydrogels for Microtissue

Landon Loving on LinkedIn: Fierce Biotech Fundraising Tracker '23

LinkedIn Landon Loving 페이지: Biotech pipeline hosts 163

Journal of Medicinal Chemistry

Hepatitis B drug developers chart slow progress, just like in hep C
Recomendado para você
você pode gostar

![Halt [DOORS] by TheWandererH on DeviantArt](https://images-wixmp-ed30a86b8c4ca887773594c2.wixmp.com/f/f5e9214b-46cf-4479-bac6-881ddf00ec6e/dfece3t-94478477-1c07-408b-ad12-407e1699f74a.jpg/v1/fill/w_1280,h_1114,q_75,strp/halt__doors__by_thewandererh_dfece3t-fullview.jpg?token=eyJ0eXAiOiJKV1QiLCJhbGciOiJIUzI1NiJ9.eyJzdWIiOiJ1cm46YXBwOjdlMGQxODg5ODIyNjQzNzNhNWYwZDQxNWVhMGQyNmUwIiwiaXNzIjoidXJuOmFwcDo3ZTBkMTg4OTgyMjY0MzczYTVmMGQ0MTVlYTBkMjZlMCIsIm9iaiI6W1t7ImhlaWdodCI6Ijw9MTExNCIsInBhdGgiOiJcL2ZcL2Y1ZTkyMTRiLTQ2Y2YtNDQ3OS1iYWM2LTg4MWRkZjAwZWM2ZVwvZGZlY2UzdC05NDQ3ODQ3Ny0xYzA3LTQwOGItYWQxMi00MDdlMTY5OWY3NGEuanBnIiwid2lkdGgiOiI8PTEyODAifV1dLCJhdWQiOlsidXJuOnNlcnZpY2U6aW1hZ2Uub3BlcmF0aW9ucyJdfQ.EmHkO-1vnNgie3PrRzW_Ip0K030MAIq6lvZbEgWdsXQ)



![Closed] Scripter! - Recruitment - Developer Forum](https://devforum-uploads.s3.dualstack.us-east-2.amazonaws.com/uploads/original/4X/d/b/9/db9ca5ad8c750c1d49743f66d1859c69fad9bef2.gif)


:max_bytes(150000):strip_icc()/jack-black-1fcf46f7692047d19c408a1783d0ff34.jpg)





