Ethide Laboratories - USP 88 In-Vivo Cytotoxicity Testing
Por um escritor misterioso
Last updated 10 novembro 2024

Learn what USP 88 cytotoxicity tests are available and which ones you will need to meet the regulatory requirements for your medical devices.

Half-Sandwich” Ruthenium Complexes with Alizarin as Anticancer Agents: In Vitro and In Vivo Studies

Vaccine Delivery TechnologyMethods and Protocols - 1071607944 PDF, PDF, B Cell
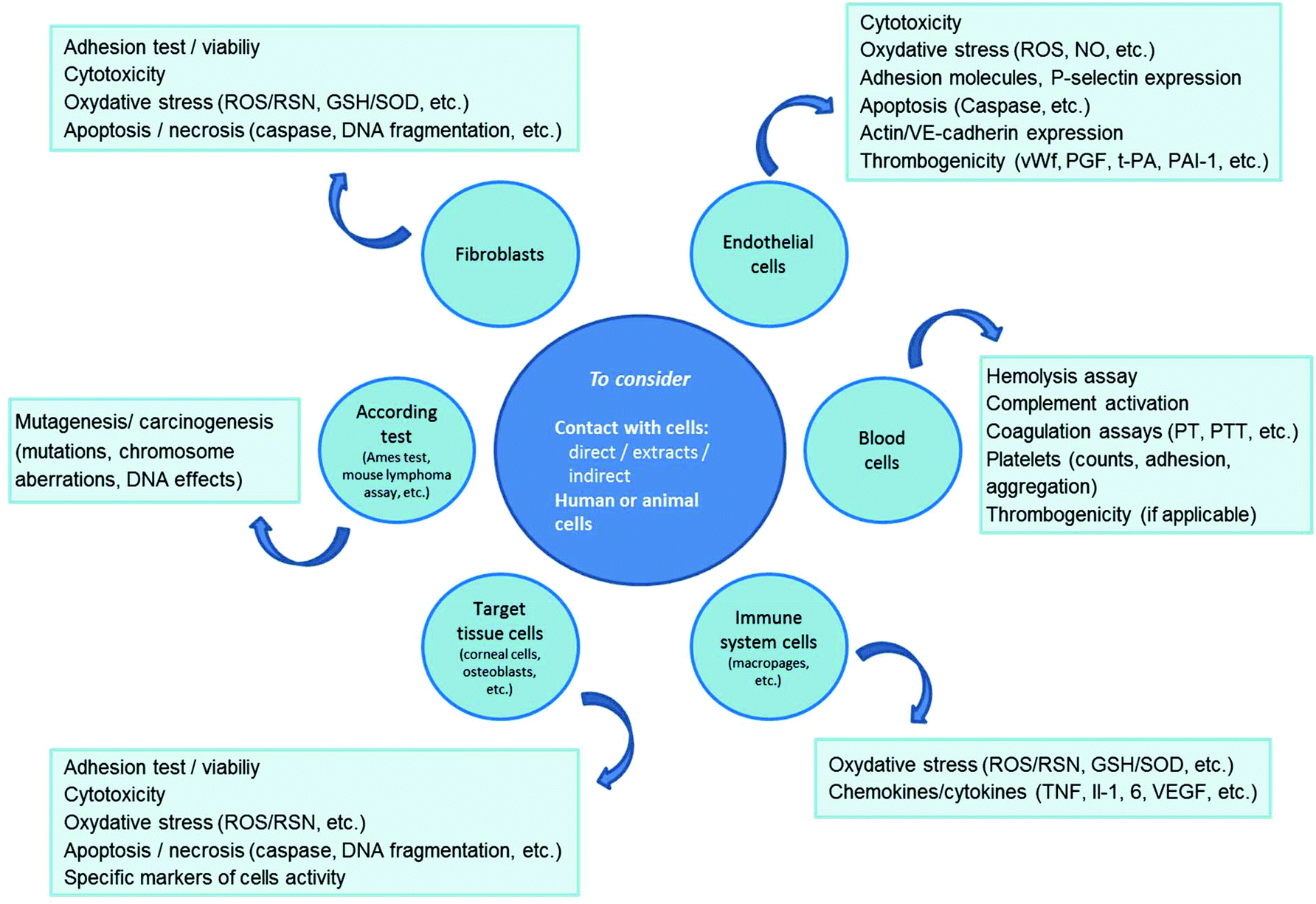
Biocompatibility of polymer-based biomaterials and medical devices – regulations, in vitro screening and risk-management - Biomaterials Science (RSC Publishing) DOI:10.1039/C8BM00518D
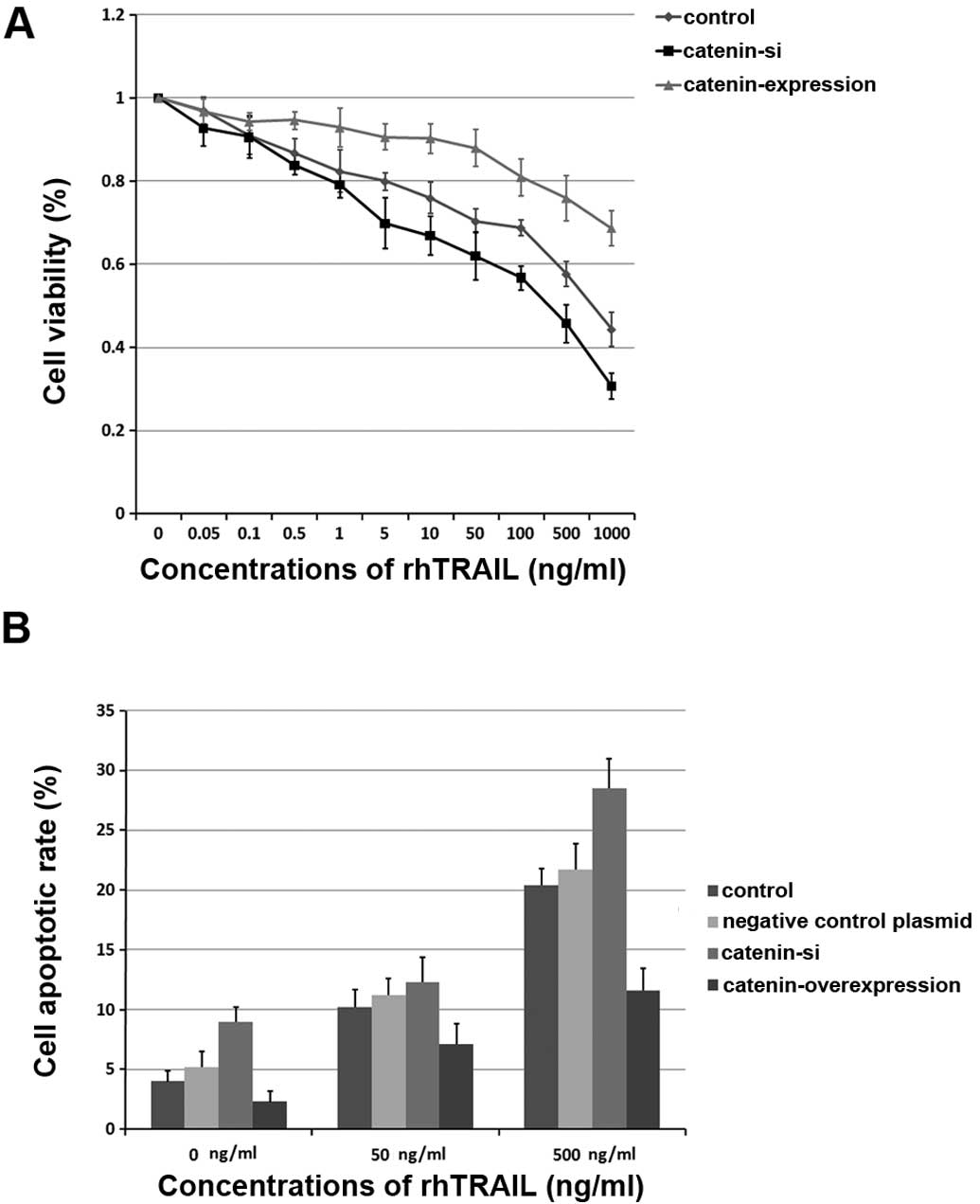
β-catenin is regulated by USP9x and mediates resistance to TRAIL-induced apoptosis in breast cancer

In-vivo & in-vitro toxicity test of molecularly engineered PCMS: A potential drug for wireless remote controlled treatment - ScienceDirect

Integrating Emerging Polymer Chemistries for the Advancement of Recyclable, Biodegradable, and Biocompatible Electronics - Chiong - 2021 - Advanced Science - Wiley Online Library
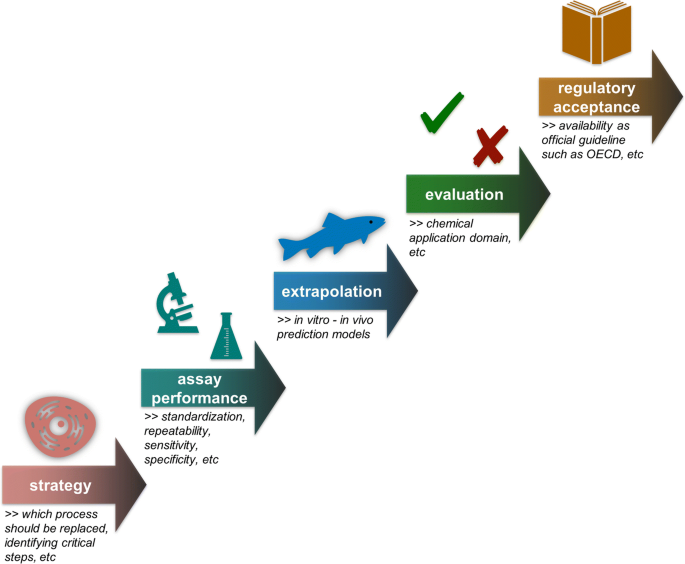
In vitro or not in vitro: a short journey through a long history, Environmental Sciences Europe

SciELO - Brasil - The Relationship Between Neuroprotective Activity and Antigenotoxic and Acetylcholinesterase Inhibitory Effects of Glaucium corniculatum Extracts The Relationship Between Neuroprotective Activity and Antigenotoxic and

Cell-mediated Cytotoxicity Assay: Total Cytotoxicity Test

Cell Cytotoxicity Assay, Cell Toxicity Assay

Repopulation of a 3D-simulated periapical lesion cavity with triggered osteoblastic-differentiated dental pulp stem cell spheroids
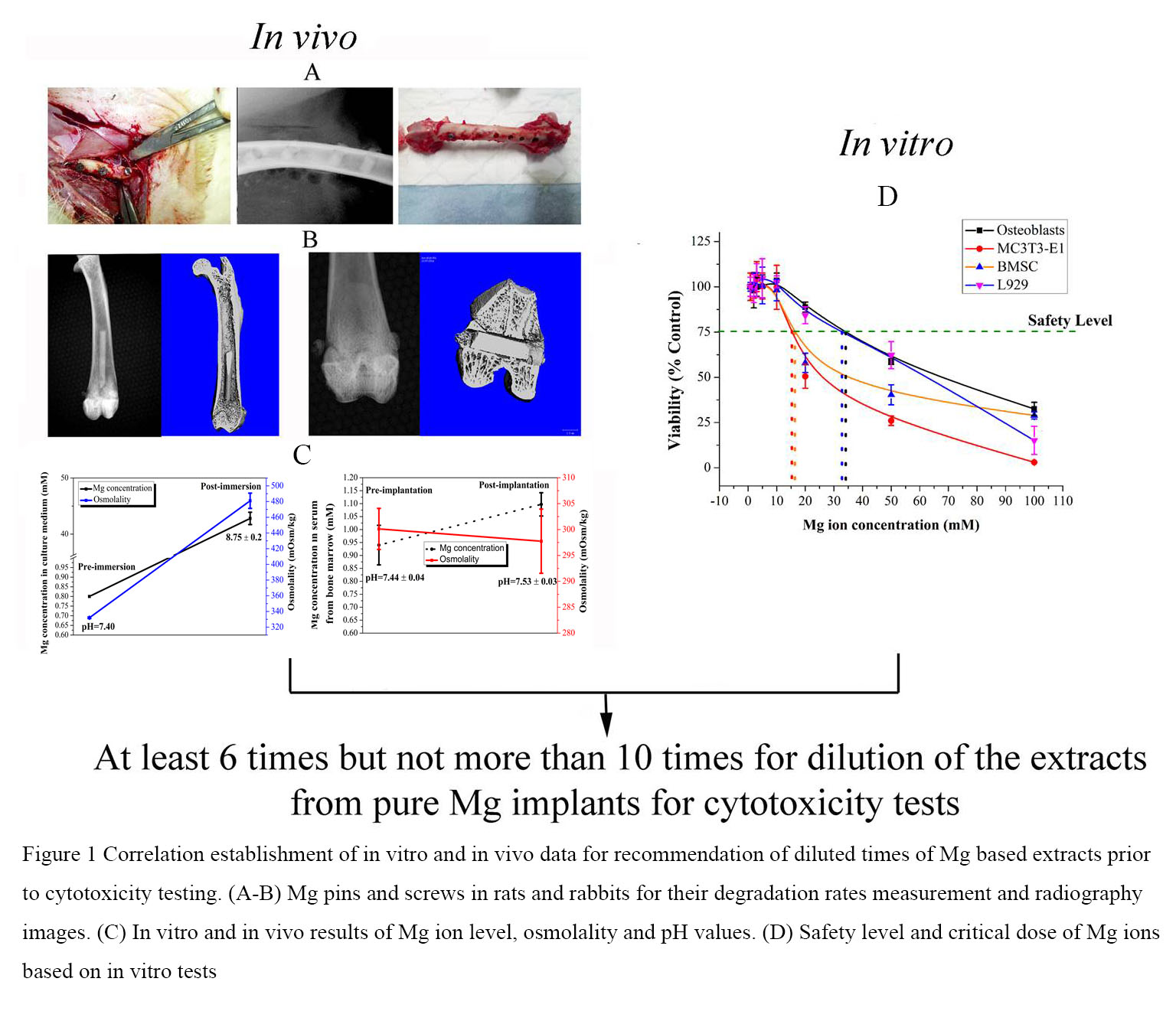
Frontiers Modified cytotoxicity test protocol for Mg metals developed for class III medical devices
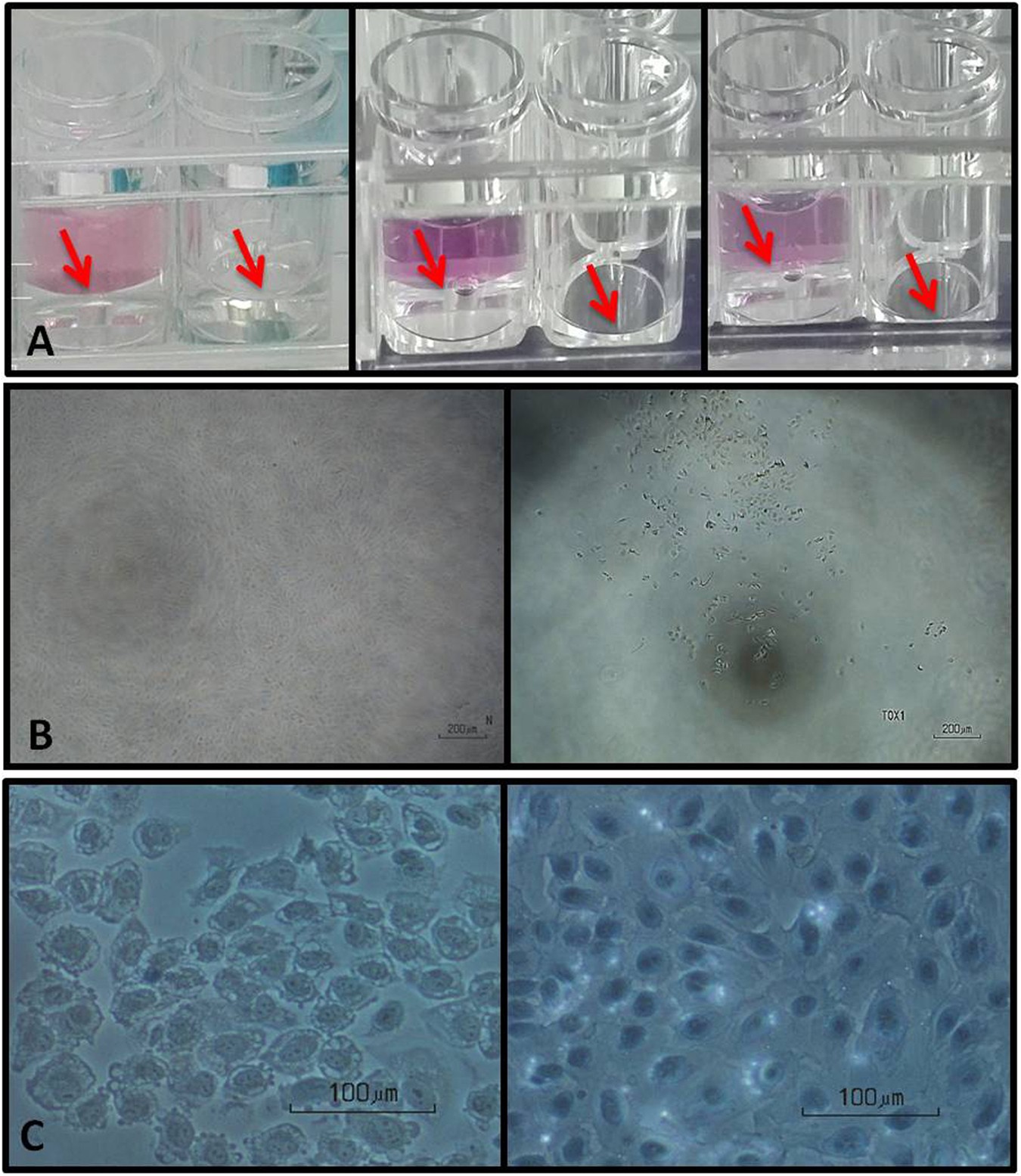
Comparison between direct contact and extract exposure methods for PFO cytotoxicity evaluation
Recomendado para você
você pode gostar





:max_bytes(150000):strip_icc()/iStock_000021026431_Large-56a5c79b3df78cf77289db7d.jpg)