FDA's Fast-Track for Rexulti Raises Concerns
Por um escritor misterioso
Last updated 12 novembro 2024

CMS efforts to reduce use of unnecessary antipsychotics in nursing homes may conflict with marketing efforts for the drug.

Not Everyone Agreed With FDA Approval of Antipsychotic Rexulti for Agitation
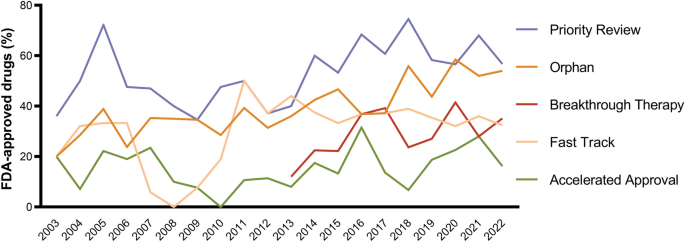
Special FDA designations for drug development: orphan, fast track, accelerated approval, priority review, and breakthrough therapy
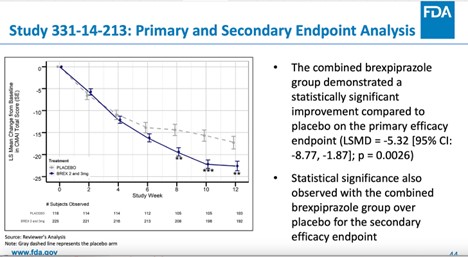
Not Everyone Agreed With FDA Approval of Antipsychotic Rexulti for Agitation - Mad In America

Rare Disease Clinical Trials: Strategies Learned from Duchenne Muscular Dystrophy

FDA rushes approval of dementia drug that quadruples risk of death
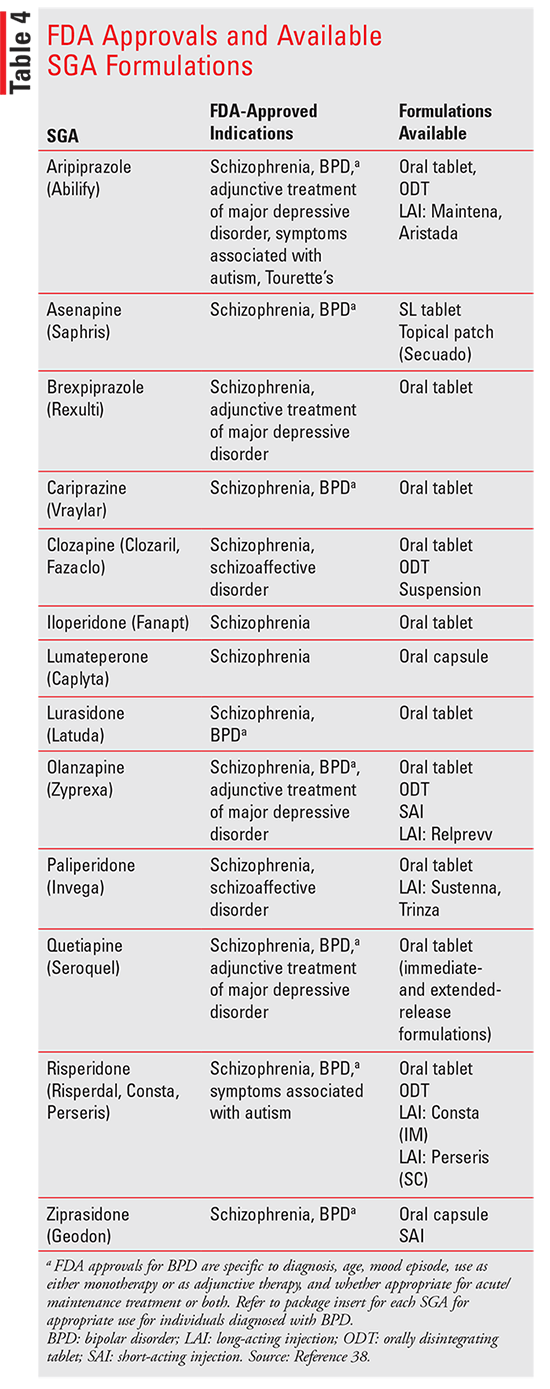
Lesson: Assessing the Current Antipsychotics Landscape

Alzheimer's drug controversies and scandals: behind closed doors

FDA's Fast-Track for Rexulti Raises Concerns
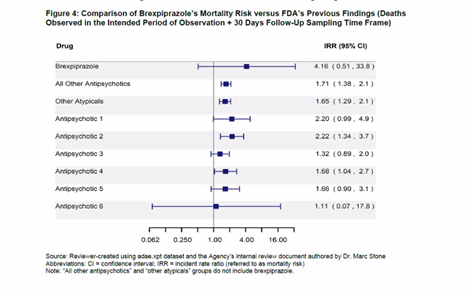
Not Everyone Agreed With FDA Approval of Antipsychotic Rexulti for Agitation - Mad In America

FDA approves supplemental new drug application for Rexulti to treat Alzheimer's agitation

FDA's accelerated drug approvals often lack confirmatory evidence : Shots - Health News : NPR

REXULTI® (brexpiprazole)

Rexulti Review Effective for Schizophrenia and Depression? – Illuminate Labs
Recomendado para você
você pode gostar












![FNF VS Demae Channel [FULL WEEK] [Friday Night Funkin'] [Mods]](https://images.gamebanana.com/img/ss/mods/60f7ffd41fbbc.jpg)

![comm] Pokemon Xenoverse Family - Amber by Desinho on DeviantArt](https://images-wixmp-ed30a86b8c4ca887773594c2.wixmp.com/f/cc710b17-c9b4-4afb-a166-6c1f9ca1554c/df9a51y-dda6d2e6-ca8a-4a87-bce3-e22ac70071a1.png/v1/fill/w_1280,h_1068,q_80,strp/_comm__pokemon_xenoverse_family___amber_by_desinho_df9a51y-fullview.jpg?token=eyJ0eXAiOiJKV1QiLCJhbGciOiJIUzI1NiJ9.eyJzdWIiOiJ1cm46YXBwOjdlMGQxODg5ODIyNjQzNzNhNWYwZDQxNWVhMGQyNmUwIiwiaXNzIjoidXJuOmFwcDo3ZTBkMTg4OTgyMjY0MzczYTVmMGQ0MTVlYTBkMjZlMCIsIm9iaiI6W1t7ImhlaWdodCI6Ijw9MTA2OCIsInBhdGgiOiJcL2ZcL2NjNzEwYjE3LWM5YjQtNGFmYi1hMTY2LTZjMWY5Y2ExNTU0Y1wvZGY5YTUxeS1kZGE2ZDJlNi1jYThhLTRhODctYmNlMy1lMjJhYzcwMDcxYTEucG5nIiwid2lkdGgiOiI8PTEyODAifV1dLCJhdWQiOlsidXJuOnNlcnZpY2U6aW1hZ2Uub3BlcmF0aW9ucyJdfQ.r2SvIDXL_5o5C-L-X2cRhU08eY9cCHej5Ybus3CE_mM)


